Daclatasvir and Sofosbuvir: Hepatitis C Treatment
In the landscape of chronic hepatitis C treatment, the advent of the daclatasvir and sofosbuvir combination marks a pivotal shift towards a more effective and tolerable regimen. This introduction outlines the significance of their synergistic action in combating one of the most challenging liver diseases, emphasizing the blend of innovation and patient-centric care.
Mechanism of Action
Daclatasvir and sofosbuvir combine two potent mechanisms targeting the hepatitis C virus (HCV). Daclatasvir inhibits the NS5A protein, a crucial component for viral RNA replication and virion assembly. Sofosbuvir, a nucleotide analog, inhibits NS5B polymerase, responsible for the RNA replication of the virus. Together, they offer a dual blockade against HCV replication, enhancing the chances of achieving sustained virological response (SVR).
Indications for Use
This combination is indicated for the treatment of adult patients with chronic hepatitis C virus (HCV) infection across genotypes 1, 2, 3, 4, 5, and 6. It is suitable for those with or without cirrhosis, including patients with compensated cirrhosis (Child-Pugh A) and in certain cases, those with decompensated cirrhosis (Child-Pugh B or C).
Available Formulations
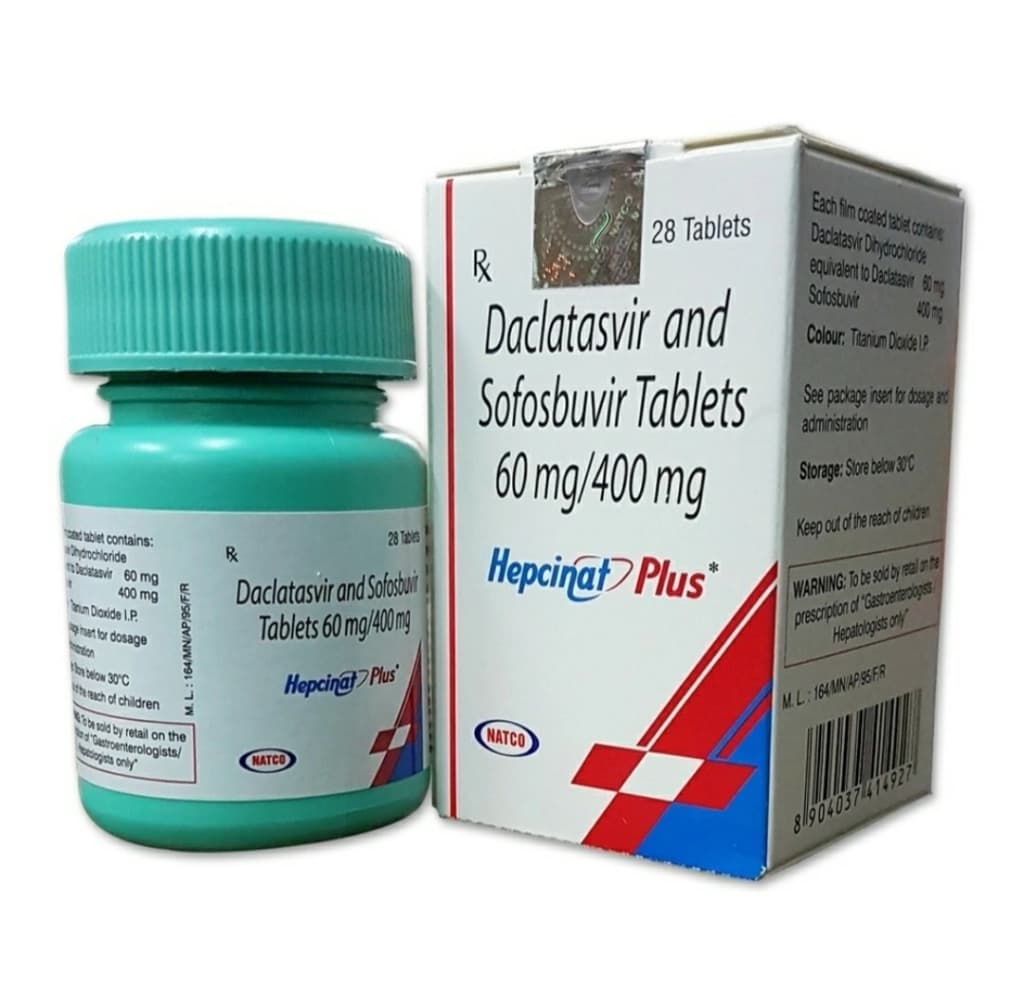
The treatment is available as a co-formulated oral tablet containing 400 mg of sofosbuvir and 60 mg of daclatasvir. This fixed-dose combination simplifies the regimen, enhancing patient adherence.
Recommended Dosages
The standard dosage for adults is one tablet (400 mg sofosbuvir/60 mg daclatasvir) taken orally once daily. For patients without cirrhosis or with compensated cirrhosis, the recommended treatment duration is 12 weeks. For those with decompensated cirrhosis, a 24-week treatment may be recommended based on the patient’s genotype and clinical evaluation.
Contraindications and Side Effects
Contraindications include patients with hypersensitivity to daclatasvir or sofosbuvir. Common side effects are fatigue, headache, nausea, and insomnia. Less commonly, patients may experience severe bradycardia when co-administered with amiodarone. Hepatitis B reactivation has been reported in co-infected patients; thus, monitoring is advised.
Special Considerations
Caution is warranted when prescribing this combination to patients co-infected with the hepatitis B virus due to the risk of HBV reactivation. Drug interactions should be carefully managed, especially with inducers or inhibitors of CYP3A4 and P-gp, which can affect daclatasvir levels, and with drugs affecting sofosbuvir metabolism.
Administration Advice
The co-formulated tablet should be taken whole with or without food. If a dose is missed and it is within 18 hours of the missed dose, take it as soon as remembered. If over 18 hours have passed, skip the missed dose and take the next dose at the regular time.
Pediatric Considerations
For pediatric patients, the use of sofosbuvir and daclatasvir has been less extensively studied, and dosages may need to be adjusted. Currently, separate formulations of sofosbuvir and daclatasvir are available for children, allowing for dosing adjustments based on weight and age.
Storage Guidelines
Store the tablets at room temperature, away from direct light and moisture. Keep the medication in its original bottle, tightly closed when not in use, and out of reach of children and pets to avoid accidental ingestion.
Comparative Analysis
| Feature | Daclatasvir and Sofosbuvir | Other Treatments |
|---|---|---|
| Mechanism of Action | Targets NS5A and NS5B proteins | May target different viral proteins |
| Spectrum of Activity | Effective across genotypes 1, 2, 3, 4, 5, 6 | Genotype-specific effectiveness |
| Treatment Duration | 12 weeks for most cases, 24 weeks for specific conditions | Varies, often longer |
| Side Effects Profile | Generally well-tolerated; common side effects include fatigue and headaches | Often more severe side effects |
| Drug Interactions | Specific interactions requiring caution | Varied interaction profiles |
| Ease of Administration | Single daily oral tablet | May require complex regimens |
This table underscores the unique advantages of daclatasvir and sofosbuvir, highlighting its broad-spectrum activity, manageable side effect profile, and simplified treatment regimen compared to other therapies.
Conclusion
The daclatasvir and sofosbuvir combination represents a significant milestone in the fight against chronic hepatitis C. By offering a simplified, yet highly effective treatment regimen, it not only enhances patient adherence but also paves the way for more accessible care. With its broad-spectrum efficacy and manageable safety profile, this combination sets a new standard in antiviral therapy, embodying the progress in medical science’s quest to combat viral diseases. As we move forward, the continued study and application of such treatments will undoubtedly play a crucial role in the global effort to reduce, and ultimately eliminate, hepatitis C as a public health threat.