Atorvastatin and Liver Damage: Risks Explained
Atorvastatin is recognized as a powerful and highly effective medication designed to combat high cholesterol levels. It operates by selectively inhibiting the enzyme hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which is pivotal in the body’s cholesterol production pathway. As part of the statin class, atorvastatin plays a vital role in reducing total serum cholesterol and low-density lipoprotein (LDL) levels. This reduction is crucial in decreasing the likelihood of atherosclerosis and its potentially fatal complications, such as myocardial infarction (heart attack) and stroke.
What about Atorvastatin Liver Damage?
Gaining approval for medical use in the United States in 1996, atorvastatin has swiftly become a staple in the pharmaceutical landscape, with over 50 million prescriptions issued annually. Its primary use is for the management of hypercholesterolemia in individuals facing a significant risk for heart, brain, and peripheral vascular diseases. Marketed under both its generic name and the brand name Lipitor, and also in combination with cardiovascular medications like amlodipine (Caduet), atorvastatin is dispensed in dosages of 10, 20, 40, and 80 mg. The dosage is tailored to the individual, ranging from 10 to 80 mg daily, based on the patient’s tolerance and specific lipid profile requirements. While the drug is celebrated for its efficacy, it is accompanied by potential side effects, including, but not limited to, muscle cramps, headaches, joint pains, abdominal pain, nausea, and general weakness—commonalities shared with other statins. Rare, yet serious side effects may involve liver damage, myopathy, rhabdomyolysis, and immune-mediated necrotizing myopathy, highlighting the need for cautious administration.
Understanding Atorvastatin’s Impact on Cholesterol and Heart Health
By significantly lowering the levels of harmful cholesterol, atorvastatin contributes to the prevention of plaque buildup in the arteries, a condition known as atherosclerosis. This process is instrumental in reducing the risk of severe cardiovascular events, emphasizing the drug’s role in promoting heart health and longevity. The widespread prescription of atorvastatin underscores its importance in the preventative healthcare regimen, particularly for patients with existing risk factors for cardiovascular diseases.
The Pharmacological Mechanism of Atorvastatin
Atorvastatin’s mode of action involves the inhibition of the HMG-CoA reductase enzyme, a key catalyst in the mevalonate pathway of cholesterol biosynthesis. By blocking this enzyme, atorvastatin effectively reduces the production of cholesterol in the liver, leading to a decrease in the overall levels of cholesterol within the body. This mechanism not only lowers LDL cholesterol but also contributes to a reduction in triglycerides and a modest increase in high-density lipoprotein (HDL) cholesterol, further benefiting cardiovascular health.
Atorvastatin and Hepatotoxicity: A Closer Look
The Spectrum of Liver-Related Side Effects: What about Atorvastatin Liver Damage?
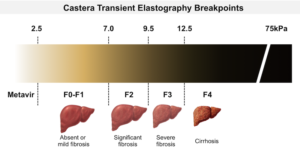
Atorvastatin therapy, although generally safe, is associated with a spectrum of liver-related side effects ranging from mild, asymptomatic elevations in serum aminotransferases to rare instances of clinically significant liver injury. Mild elevations in liver enzymes are observed in 1% to 3% of patients, typically without symptoms and resolving spontaneously. However, attention is warranted when enzyme levels exceed three times the upper limit of normal, an occurrence noted in less than 1% of patients but with higher incidences linked to increased dosages.
Navigating Hepatotoxicity with Atorvastatin
Clinically apparent liver injury from atorvastatin, although a rare occurrence, presents a spectrum of manifestations and considerations for both patients and healthcare providers. These instances are estimated to happen between 1:3000 to 1:5000 individuals treated with atorvastatin. The range of hepatotoxicity symptoms can significantly vary, marking the complexity and the need for careful monitoring during treatment. Here are key points to understand the nature and management of liver injury associated with atorvastatin:
- Incidence Rate: The likelihood of experiencing clinically significant liver injury from atorvastatin is relatively low, with estimates suggesting an occurrence rate of between 1 in 3000 to 1 in 5000 treated patients. This rarity underscores the overall safety profile of the drug when used as directed;
- Variability in Manifestations: Liver injury due to atorvastatin can present in various forms, ranging from mild cholestatic hepatitis to more severe hepatocellular damage. This variability necessitates a broad understanding of potential liver-related side effects for accurate diagnosis and management;
- Onset of Symptoms: The onset of liver injury symptoms typically falls within six months after starting atorvastatin therapy or following a dose adjustment. This timeframe is critical for healthcare providers to monitor liver enzyme levels closely, especially in the initial stages of treatment or after increasing the dosage;
- Resolution of Symptoms: Despite the potential severity of hepatotoxicity, the majority of cases are self-limiting or resolve entirely upon discontinuation of atorvastatin. This characteristic is comforting for both patients and physicians, indicating that monitoring and timely intervention can effectively mitigate the risk of long-term liver damage.
Understanding these aspects of atorvastatin-associated hepatotoxicity is crucial in optimizing patient care, ensuring that the benefits of cholesterol reduction are achieved with minimal risk to liver health. The approach to managing atorvastatin therapy involves a balance of vigilant monitoring, recognizing the signs of liver injury early, and taking appropriate action to prevent or limit damage, thereby maintaining the integrity of patient health while effectively managing cholesterol levels.
Autoimmune Hepatitis Triggered by Atorvastatin
A subset of hepatocellular injury cases exhibits autoimmune features, such as elevated immunoglobulin levels and positive antinuclear antibodies (ANA), with liver biopsy findings consistent with autoimmune hepatitis. These cases often respond to cessation of atorvastatin, although corticosteroid therapy may be necessary for resolution. Intriguingly, a few cases persist beyond the discontinuation of atorvastatin, necessitating long-term immunosuppressive treatment. The precise nature of the relationship between statin therapy and autoimmune hepatitis remains an area of ongoing research and debate.
Conclusion
Atorvastatin has cemented its role as a cornerstone in the management of high cholesterol and the prevention of cardiovascular diseases. Despite its proven benefits, the potential for liver-related side effects, although rare, necessitates vigilant monitoring and individualized patient care. The balance between the drug’s substantial benefits in reducing cardiovascular risk and the low incidence of serious adverse effects underscores the importance of atorvastatin in contemporary medical practice.
FAQs:
If you notice symptoms that might indicate liver problems, such as unusual fatigue, yellowing of the skin or eyes (jaundice), dark urine, or severe abdominal pain, it’s crucial to contact your healthcare provider immediately. Your doctor may recommend undergoing liver function tests to assess your liver enzyme levels and determine the appropriate course of action. This could involve adjusting your atorvastatin dosage or discontinuing the medication, depending on the severity of the symptoms. It’s important to never stop taking atorvastatin without consulting your healthcare provider, as they will provide guidance based on your specific situation and health condition.
Guidelines suggest that liver enzyme tests should be conducted before starting atorvastatin therapy and as clinically indicated thereafter. Although routine monitoring of liver enzymes in all patients is no longer universally recommended, it’s important for those starting atorvastatin or undergoing dose increases to have their liver function checked. If you have a history of liver disease, or if you consume significant amounts of alcohol, more frequent monitoring may be advised. Your healthcare provider will determine the monitoring schedule based on your overall health, risk factors, and response to the medication.
While atorvastatin-related hepatotoxicity is rare, adopting a healthy lifestyle may help minimize the risk of liver issues. This includes maintaining a balanced diet, regular exercise, avoiding excessive alcohol consumption, and not using illegal drugs. Additionally, inform your healthcare provider about all medications, supplements, and herbal products you are taking, as certain substances can interact with atorvastatin and affect liver health. Regular check-ups and open communication with your healthcare provider can also ensure that any potential liver-related issues are identified and managed promptly, allowing for a safer and more effective atorvastatin treatment regimen.